Unit 8 - Oxidative Phosphorylation Flashcards
(35 cards)
What is oxidative phosphorylation?
The formation of ATP as a result of the transfer of eldctrons from NADH and FADH2 to O2 by carrier in the ETC
What is the core idea of oxidative phosphorylation?
Create a proton gradient using the high electron transfer potential of the NADH and FADH2 carriers to create a electrochemical gradient from the IMM into the mitochondrial matrix. This create a Proton moving force that drives ATP synthase to create ATP
What are the 3 ways in which electrons can be transfered as?
Free electrons
hydrogen atoms
H- (hydride ions)
How can we measure reduction potential?
Using standard reduction potentials - the more negative, the more willing something is to reduce/accept electrons
How many ATP molecules can we produced from NADH in theory? In practice?
In theory, 7
In practice, 2.5 ATP/per NADH
What is complex 1 of the ETC, and what does it do?
NADH-Q Oxidreductase
NADH is reduced, giving 2e- (accepted by FMN in oxidreductase)
Electrons transfered to FE-S clusters, and finallly to co-enzyme Q (Which is reduced); i.e. ubiquinone to ubiquinole
This pumps 4 protons into IMS from matrix
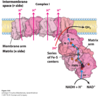
Net equation of Complex 1?

What is complex 2 and what does it do?
Succinate-Q oxidreductase (AKA succinate Q reductase)
Succinate dehydrogenase, from Kreb cycle, is part of this complex
Succinate is Oxidized (hence it’s first)
electrons transfered via FADH2 within the complex
Q reduced to QH2 (ubiquintine to ubioquinol via FeS clusters)
NO protons are pumped
What is coenzyme Q? (i.e. structure)
What does it do?
Small hydrophobic molecule in IMM, contains repeated isoprenoid tail (type of hydrocarbon)
Accepts 2e- to reduce to ubiquinole (from ubiquinone)
Acts as electron shuttle for electrons from complex I and II & carries to complex III
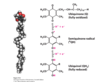
What is complex III and what does it do?
Q-cytochrome C oxidreductase
Takes 2 electrons from QH2 (oxidation) and transfers it individually to FeS clusters to heme C1 then to cytochrome c (reduction)
Pumps 4 protons into IMS
2 come from QH2 (Which came from the matrix going further back)
2 come from matrix
What are the 2 key cytochromes in complex III?
cyt b
cyt c1
What is the net equation of the complex III reaction?

What is cytochrome c’s prupose? structure? Qualities? Location?
Electron shuttle, carrying electrons from complex III to IV
Contains a iron heme group, is water soluble, and carries 1 electron total
Sits on the Inter-membrane space side of the IMM
What is complex IV? What are the functions of complex IV?
Performs the final reduction of oxygen using electrons from cytochrome c
Prevents partially reduced oxygen (i.e. Reactive Oxygen Species, or oxygen radicals essentialy) from being released)
Pumps 2 electrons
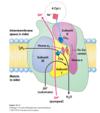
What is the structure of complex IV? What does it contain?
Contains 2 cytochromes, cytochromes a and a3
Contains 2 copper centres; CuA and CuB
Oxygen binds between heme a3 and CuB
What is the net equation for the complex IV reaction?
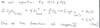
What are examples of toxic substances that block the ETC?
N3 (azide), CO, CN- (cyanide)
What is F0
One of the 2 ATP synthase subunits
Imbedded in IMM
Contains H+ channels
Subunits use ROMAN naming system

What is F1
Second ATP synthase subunit
Extends into matrix
Synthesizes ATP when linked to H+ in F0
Subunits use GREEK naming system
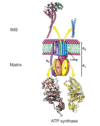
What is the structure of F1 subunit
Has 3αß subunits arranged in a ball around the gamma subunit
Gamma unit spins from F0 subunit activity, causing αß subunits to change between Open, Loose, and Tight site conformations
Open allows ATP and ADP to leave, Loose allows ADP and Pi to enter but not leave, and Tight sights creates ATP but retains it.
Cyclical action generates ATP

How much ATP is synthesized per proton? Per NADH? FADH2? What is the rationalle behind it?
4H+/ATP is best estimate;
3H+ to spin ATP synthase
1 H+ to move Pi
Thus:
10/4=2.5 NADH/ATP
6/4 = 1.5 ATP/FADH2
What is the structure of subunit c of F0?
- Composed of 2 α-helices
- Span IMM
- 10-12 cylindrical shape
- 1/2 down one of the helixes is a Aspartic acid residue that can be protonated/deprotonated
- Entire subunit can rotate
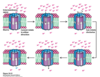
What is the structure of subunit a of F0?
- Stationary
- Has a “clamp” that cover 2 of the c subunits
- Has 2 channels; one channel is open to the IMS and goes halfway down subunit a, while the other is open to the matrix and goes halfway up subunit a
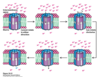
Which way does F0 spin?
Clockwise; such that proton goes through IMS 1/2 channel, forms uncharged aspartic acid, rotates around, and enters matrix 1/2 channel where subunit c deprotonates back into asparatate







