Physiology of Neurons Flashcards
(54 cards)
Electrical Synapses
What is the clinical importance of measuring conduction velocity?
- This is measured to investigate the source of motor weakness in arms and legs.
- It can detect gross pathological changes such as conduction block and conduction slowing, which could signify demyelination or degeneration.
- It can help diagnose: Peripheral neuropathy, Carpal tunnel syndrome, Spinal disc herniation, etc.
Draw a graph of a neural action potential. Include axes (and their labels), units, and approximate numerical values.

For each of those phases of the action potential: list the ionic current (and the ion’s direction of movement) that is responsible.
- Resting membrane potential is determined primarily by K+ moving down its concentration gradient (leaking out of cell), leaving behind negative anions inside membrane. This is due to leak and some inward rectifier currents.
- Depolarisation is due to Na+ rapidly coming into the cell.
- The threshold is the voltage above which the cell is committed to completing an action potential. It occurs when inward Na+ current depolarizes the cell faster than outward K+ current can repolarise the cell back to the RMP.
- Repolarisation is due to Na+ channels inactivating while K+ is rapidly leaving the cell via the delayed rectifier channels. It is NOT the Na/K pump, which only changes Vm by 3 mV.
- The afterhyperpolarisation is when Vm is most close to EK at the end of the AP. Many different sources of K+ permeability cause K+ to flow out: inward rectifiers, leak, and delayed rectifiers (which are delayed in their closing). In addition, during the AHP unusually little Na+ is flowing because the Na+ channels are inactivated (which occurs after a delay when Vm is above -40 mV)
Name the main transmembrane forces on an ion.
•Chemical (diffusional) and electrical
What is a graded potential?
- It is a change in the membrane potential that is NOT an action potential. It can vary in voltage amplitude and in time duration. A graded potential is not amplified, and it is not all-or-none.
- It can occur at receptor cells (eg rods and cones) where it is important in transduction of light/pressure into a graded electrical potential
- Also occurs at synapses.

What is a refractory period?
- A refractory period is a time duration after one action potential has just fired when another action potential either cannot (absolute refractory period) is resistant to (relative refractory period) restimulation to begin a new action potential. It often is associated with Vm being lower than the RMP (the after-hyperpolarisation), and it is usually caused by a high permeability to K+ (i.e. more K+ channels are open than at rest).
What is an equilibrium potential?
- It is the voltage where the amount of a particular ion (e.g. Na+) flowing out of the cell = the amount of that ion flowing in. It happens when the electrochemical forces for that ion type are in equilibrium.
- E.g. For Na+, this occurs when the diffusion (chemical) forces pushing Na+ into the cell equal the voltage (electrical) forces pushing Na+ out of the cell
Draw a diagram showing the two forces on K+ ions at its equilibrium potential.

Name the equilibrium potentials for Na+, K+, Ca2+ and Cl¯ (in a typical cell).
- ENa = ~ +55 mV (or you could say +60).
- EK = ~ -90 mV
- ECa = ~ +123 mV
- ECl = ~ -40mV (although this varies by cell type and is sometimes quoted as being -65 mV)
Why is the resting membrane potential -70 mV when there are so many positive K+ ions inside the cell?
- Resting membrane potential is determined by which ion has the most conductance. Because at rest K+ is by far the most conducted ion, the high K+ that is leaking out of the cell leaves behind negative ions (esp. proteins and Cl-). The fact that there are so many K+ ions inside the cell does not make the inside positive because virtually every single one of the K+ ions is balanced by a negative charge (proteins & Cl-) right next to it.
Give two examples of drug classes that block sodium channels as part of their mechanism of action.
- Local anaesthetics (eg lidocaine). Some anticonvulsants (eg carbamazepine). Type I cardiac antiarrhythmic drugs (eg quinidine, phenytoin and lidocaine (which is also classed as a class Ib antiarrhythmic).
Name three all-or-none physiological phenomena characterised by positive feedback. Hint: many examples are in the endocrine/reproductive systems from A levels.
- •Parturition (giving birth). Menstrual cycle (ovulation). Vomiting. The action potential. Blood clotting cascade.
Draw a diagram showing how depolarisation is a positive feedback loop.
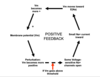
Name two kinds of synapses.
- Chemical synapses, electrical synapses. Also inhibitory and excitatory.
What is the conduction velocity for an alpha motor neuron?
- 100 meters/second
What is the conduction velocity for C fibres for pain?
- 1 meter/second
What are the structural differences between alpha motor neurons and C fibres responsible for the difference in their conduction velocity? Explain which structural features increase and which decrease conduction velocity.
- Alpha is large diameter and myelinated, both of which increase conduction velocity.
- Myelination increases transmembrane resistance, which leads to more efficient electrotonic signalling inside the axon between nodes of Ranvier.
- Increased diameter leads to increased conduction velocity because intracellular resistance along the length of the axon is lower. This leads to more efficient (hence faster) electrotonic transmission.
Why do neurons with a larger cross sectional diameter conduct faster?
- Larger diameter means that along the length of the intracellular fluid of the axon there is less resistance and higher conductance.
- Using the river rapids metaphor, a wider river (higher conductance) leads to more flow (current) for the same drop in height (voltage)
Why does myelination lead to increased conduction velocities?
- Myelination increases the transmembrane resistance and decreases the transmembrane capacitance.
What are the Nodes of Ranvier and what is their purpose?
- The nodes of Ranvier are thin, unmyelinated strips of membrane along the length of a myelinated axon.
- The nodes are excitable, the axon under myelin is not
- The nodes have clusters of Na+ channels
- The “bare” cell membrane at the node is specialised for detecting a distant transmembrane electric field (electrotonically) and then firing an action potential in response to it.
- Nodes of Ranvier are essential to increasing conduction velocity by having action potentials “jump” from one node to the next – so-called saltatory conduction.
- Electrotonic transmission is much faster than starting an action potential, but electrotonic transmission is limited by distance because the signal gets smaller as it travels further from its origin
Give an example of a local anaesthetic and explain how they work.
- Lidocaine (lignocaine). These block sodium channels, which raises the threshold of action potentials and thus lowers local excitability
How are electrical synapses molecularly coupled?
- Via gap junctions
Name 5 ways by which chemical and electrical synapses differ. (do not include duplicate or trivial observations)
- Electrical synapses are: faster, bidirectional, 10-fold thinner gap, no amplification, no plasticity (and thus no learning) *E


